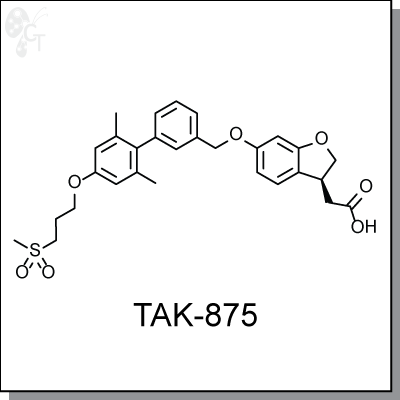
TAK-875 is an orally-available, benzofuran-based, agonist of GPR40 for the potential treament of Type 2 diabetes mellitus, with an human EC50 of 0.014 uM in a FLIPR assay. It has high selectivity over GPR41, GPR43, and GPR120, with EC50s all greater than 10 uM. [1] Oral dosing (0.3-3 mg/kg) of TAK-875 in a glucose intolerance test in female Wistar rats reduced blood glucose excursion. Insulin secretion was increased during an oral glucose tolerance test.
TAK-875 was shown to activate the Gqa-mediated signalling pathway in pancreatic b-cells. Prolonged agonist stimulation by TAK-875 revealed no evidence of b-cell dysfunction or toxicity, nor does it cause a induction of a marker of apoptosis in pancreatic b-cells. [2]
Technical information:
| Chemical Formula: | C29H32O7S.1/2H2O | |
| CAS #: | 1000413-72-8 | |
| Molecular Weight: | 533.63 | |
| Purity: | >98% | |
| Appearance: | White | |
| Chemical Name: | [(3S)-6-({2',6'-dimethyl-4'-[3-(methylsulfonyl)propoxy]biphe-nyl-3-yl}meth-oxy)-2,3-dihydro-1-benzofuran-3-yl]acetic acid hemi-hydrate | |
| Solubility: | Up to 100mM in DMSO | |
| Synonyms: | TAK-875, TAK875 |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Negoro et al., ACS Med. Chem. Lett. 2010, 1, 290-294. |
| 2. | Tsujihata et al., TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J. Pharmacol. Exp. Ther. 2011, 339(1), 228-237. Pubmed ID: 21752941 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.